Answer:
32.73%
Step-by-step explanation:
To solve this problem, first find the molar mass of Al(OH)₃.2H₂O
Atomic mass of Al = 27g/mol
O = 16g/mol
H = 1g/mol
Molar mass = 27 + 3(16 + 1) + 2(2(1) + 16)
= 27 + 51 + 36
= 114g/mol
Percentage composition =
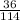 x 100 = 32.73%
x 100 = 32.73%