Density can be defined as the mass of the substance present per unit volume of that substance. Given that the masses of sugar and aluminum are equal. So, as density of a substance is inversely related to the volume, greater the density lower will be the volume occupied by same mass of a substance.
Density of sugar is 1.59
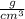
Density of aluminum is 2.70
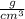
Here as the density of Aluminum is greater than that of sugar, aluminum would occupy lower volume when equal masses of aluminum and sugar are poured out.