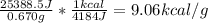Mass of olive oil burned = 0.670 g
Mass of water = 370 g
Temperature change of water =

Heat absorbed by water = Heat liberated by burning of olive oil
Calculating the heat absorbed by water:
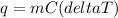
q =
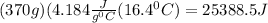
Heat released by burning of olive oil = 25388.5 J
Energy value of olive oil=