Here we have to calculate the amount of sodium hydroxide (NaOH) will react with ferric chloride (FeCl₃)
Thus 1 mole of FeCl₃ will react with 3 moles of NaOH. The integer will be 3. 0.739 g of NaOH per unit amount of FeCl₃.
Thus, 3 mol of NaOH/ 1 mol of FeCl3. 1.50 mol of NaOH per 0.50 mol of FeCl3.
The reaction between NaOH and FeCl₃ produces sodium chloride (NaCl) and ferric hydroxide [Fe (OH)₃]. The reaction is –
3NaOH + FeCl₃ = 3 NaCl + Fe(OH)₃.
The molecular weight of NaOH and FeCl₃ are 39.99 g/mol and 162.2 g/mol. As per the reaction 1 mole of FeCl₃ will react with 3 moles of NaOH.
Thus 162.2 g of FeCl3 will react with (3×39.99) = 119.97 g of NaOH. So,
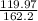 = 0.739 g per unit FeCl₃.
= 0.739 g per unit FeCl₃.