The given reaction is A---> products
Trial-1: The initial concentration of A is 0.10 M when the initial rate is 0.015 M/s
Trial-2: The initial concentration of A is 0.40 M when the initial rate is 0.060 M/s
Let the order be m
Rate law for first trial will be:
![0.015M/s = k.[0.10M]^(m)](https://img.qammunity.org/2019/formulas/chemistry/college/fw3cjd7nbgicmosw8nzi3dp7s86v931r1p.png)
Rate law for second trial will be:
![0.060M/s = k.[0.40M]^(m)](https://img.qammunity.org/2019/formulas/chemistry/college/10c56vl6ucgs287hua4bdysndgirmy9mso.png)
Rate- 2/ Rate-1 :
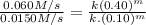
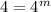
So, m = 1
Therefore the order of the reaction is 1