Answer: This electronic configuration belongs to Group 13.
Step-by-step explanation:
We are given:
The general valence electronic configuration of the elements as
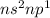 where n = 2, 3, ...7
where n = 2, 3, ...7
The number of valence electrons for this configuration are 3. This configuration belongs to p-block elements. The group to which this configuration belongs is Group 13.
The elements present in Group 13 are Boron (B), Aluminium (Al), Gallium (Ga), Indium (In), Thallium (Tl) and Ununtrium (Uut).
The green colored portion in the image belongs to Group 13.