Answer:
0.8037 g/mL is the density of ethanol.
Step-by-step explanation:
Mass of the water = m = 158 g
Volume of the water in the flask= V
Density of the water = d= 1.00 g/mL
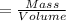
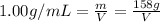
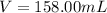
Now, mass of an ethanol hold by flask, M = 127 g
Volume of the flask = V = 158.00 mL
Density of the ethanol =D
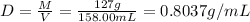
0.8037 g/mL is the density of ethanol.