Methylbut-2-ene undergoes asymmetric electrophilic addition with hydrogen bromide to produce two products:
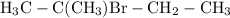 , 2-bromo-2-methylbutane;
, 2-bromo-2-methylbutane;
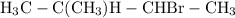 , 2-bromo-1-methylbutane.
, 2-bromo-1-methylbutane.
It is expected that
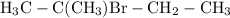 would end up being the dominant product.
would end up being the dominant product.
Step-by-step explanation
Molecules of methylbut-2-ene contains regions of high electron density at the pi-bonds. Those bonds would attract hydrogen atoms with a partial positive charge in polar hydrogen bromide molecules and could occasionally induce heterolytic fission of the hydrogen-bromide bond to produce positively-charged hydrogen ions
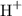 and negatively-charged bromide ions
and negatively-charged bromide ions
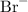 .
.
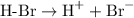
The positively-charged hydrogen ion would then attack the methylbut-2-ene to attach itself to one of the two double-bond-forming carbon atoms. It would break the pi bond (but not the sigma bond) to produce a carbocation with the positive charge centered on the carbon atom on the other end of the used-to-be double bond. The presence of the methyl group introduces asymmetry to the molecule, such that the two possible carbocation configurations are structurally distinct:
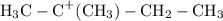 ;
;
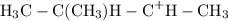 .
.
The carbocations are of different stabilities. Electrons in carbon-carbon bonds connected to the positively-charged carbon atom shift toward the electron-deficient atom and help increase the structural stability of the molecule. The electron-deficient carbon atom in the first carbocation intermediate shown in the list has three carbon-carbon single bonds after the addition of the proton
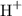 as opposed to two as in the second carbocation. The first carbocation- a "tertiary" carbocation- would thus be more stable, takes less energy to produce, and has a higher chance of appearance than its secondary counterpart. The polar solvent dichloromethane would further contribute to the stability of the carbocations through dipole-dipole interactions.
as opposed to two as in the second carbocation. The first carbocation- a "tertiary" carbocation- would thus be more stable, takes less energy to produce, and has a higher chance of appearance than its secondary counterpart. The polar solvent dichloromethane would further contribute to the stability of the carbocations through dipole-dipole interactions.
Both carbocations would then combine with bromide ions to produce a neutral halocarbon.
The position of bromine ions in the resultant halocarbon would be dependent on the center of the positive charge in the carbocation. One would thus expect 2-bromo-2-methylbutane, stemming from the first carbocation which has the greatest abundance in the solution among the two, to be the dominant product of the overall reaction.