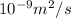Answer : The Activation energy, Ea = 201.820 KJ/mol
The Pre-exponential constant,
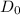 = 1.7658 ×
= 1.7658 ×
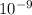
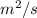
Solution : Given,
Diffusivity of Ni at
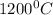 ,
,
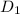 = 1.23 ×
= 1.23 ×
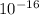
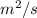
Diffusivity of Ni at
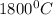 ,
,
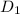 = 1.45 ×
= 1.45 ×
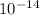
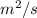
Temperature,
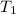 =
=
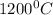 = 1200 + 273 = 1473 K
= 1200 + 273 = 1473 K
Temperature,
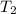 =
=
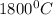 = 1800 + 273 = 2073 K
= 1800 + 273 = 2073 K
Value of R = 8.314 J/mol/K
Formula used :
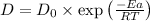 ..........(1)
..........(1)
This formula convert into logarithm term for the calculation of activation energy. So the formula is,
![ln(D_(1))/(D_(2))=(Ea)/(R)\left [(1)/(T_(2))-(1)/(T_(1)) \right ]](https://img.qammunity.org/2019/formulas/chemistry/college/821ia9e1jcam9ipte988odz61wuhpkhtec.png) ........(2)
........(2)
Now put all the values in above formula (2), we get
![ln(1.23* 10^(-16))/(1.45* 10^(-14))=(Ea)/(8.314)\left [(1)/(2073)-(1)/(1473) \right ]](https://img.qammunity.org/2019/formulas/chemistry/college/4p7bauyprmar6odohwjedmeb08fcsvy3dc.png)
Rearranging the terms, we get the value of Activation energy, (Ea) as
Ea = 201.820 KJ/mol
Now, we have to calculate the value of Pre-exponential constant,
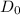 bye using formula (1)
bye using formula (1)
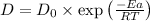
now put all the values in formula,we get
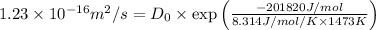
Rearranging the terms, we get the value of Pre-exponential constant,
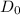 as
as
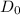 = 1.7658 ×
= 1.7658 ×