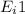 will be largest for
will be largest for
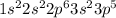 .
.
Step-by-step explanation: Ionization energy is the energy to knock off an electron from a gaseous atom of ion. First ionization energy or
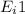 is the energy required to remove 1 loosely held electron from 1 mole of gaseous atoms to produce 1 mole of gaseous ion carrying (+)1 charge.
is the energy required to remove 1 loosely held electron from 1 mole of gaseous atoms to produce 1 mole of gaseous ion carrying (+)1 charge.
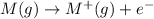
The electrons are filled according to Aufbau's rule and the orbitals which are strongly held to the nucleus follows the order
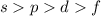 .
.
Electron is released from the outermost shell that is from the electrons which are loosely held to the nucleus, this follows the pattern
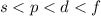 .
.
In configurations,
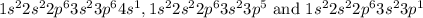
The loosely held orbital is 4s, therefore electron will be lost from that easily.
Now, in 3p orbital, one configuration has 5 electrons and one has 1 electron.
The configuration having 5 electrons will be more tightly held by the nucleus because it has more electrons that the one having only 1 electron. Hence, the electron will be lost easily from the configuration having
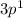 as the valence shell.
as the valence shell.
Therefore, the configuration
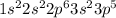 will the largest
will the largest
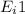 .
.