Given that the reaction is between Bismuth(III) hypochlorite and acetic acid.
Molecular equation for the reaction can be represented as:
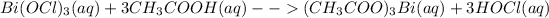
Total ionic equation for this reaction is: Bismuth(III) hypochlorite ionizes completely in solution where acetic acid being a weak acid partially ionizes in solution. On the product side, bismuth acetate being a salt completely ionizes to give 3 acetate ions and 1 bismuth ion. The other product is hypochlorous acid which ionizes partially in solution being a weak acid.
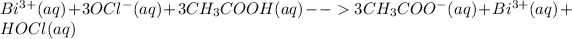
Therefore, there are 4 free ions (
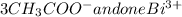 ) on the product side of the reaction.
) on the product side of the reaction.