Answer: The mass of oxygen than would react in
 is 46.6 grams
is 46.6 grams
Step-by-step explanation:
Law of multiple proportions states that when two elements combine to form two or more compounds in more than one proportion. The mass of one element that combine with a given mass of the other element are present in the ratios of small whole number.
We are given:
Mass of oxygen in carbon monoxide = 23.3 grams
The chemical formula of carbon monoxide is CO
We know that:
Molar mass of carbon = 12 g/mol
Molar mass of oxygen = 16 g/mol
In CO molecule:
If 16 g of oxygen element reacts with 12 g of carbon
o, 23.3 g of oxygen element will react with =
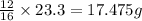 of carbon
of carbon
In
 molecule:
molecule:
Taking mass of carbon = 17.475 grams
If 12 g of carbon reacts with 32 g of oxygen
So, 17.475 g of carbon will react with =
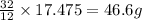 of oxygen
of oxygen
Hence, the mass of oxygen than would react in
 is 46.6 grams
is 46.6 grams