Answer : The % of (+) limonene isomer = 79%
The % of (-) limonene isomer = 0%
The % of enantiomeric excess = 58%
Explanation : Enantiomeric excess (ee) is the measurement of purity used for chiral substances.
Given,
% of pure limonene enantiomer = The % of (+) limonene isomer = 79%
Therefore, The % of (-) limonene isomer = 0%
Formula used :
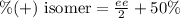
Where, ee → enantiomeric excess
Now, put all the values in above formula, we get the value of enantiomeric excess (ee).
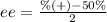
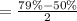
= 58%