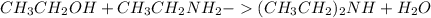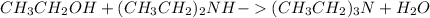Acetylene appear as gas and is unstable and thus kept in presence of other solution such as liquid ammonia.
Ethanol on reacting with ammonia produces ethylamine as follows:
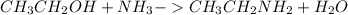
Ethanol again on reacting with ethylamine produces diethylamine and triethylamine as follows: