The initial concentration of solution is 0.0693 M. The volume of solution taken is 10 mL and it is diluted to a final volume of 500 mL.
According to dilution law, the product of initial concentration and volume is equal to the product of final concentration and volume as follows:
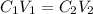
Here,
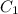 is initial concentration,
is initial concentration,
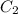 is final concentration,
is final concentration,
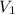 is initial volume and
is initial volume and
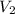 is final volume.
is final volume.
Rearranging to calculate final concentration,
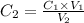
Putting the values,
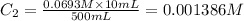
Therefore, concentration of the resulting solution is 0.001386 M.