Answer is Atomic Number of Cu.
Step-by-step explanation: For a unit cell, density can be written as
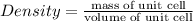
For a unit cell, Mass will be
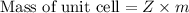
where Z= Number of atoms
m = Mass of an atom
Mass of an atom can be calculated by,
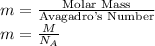
Volume of a unit cell =
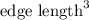
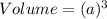
where a = edge length
Now, putting Mass and Volume of an atom in density, the equation becomes
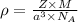
For the calculation of Avagadro's number,
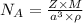
we can see from the equation, we need
1. Molar mass of atom
2. Density of unit cell
3. Edge length of the unit cell
4. Number of atoms occupying one unit cell.
Hence, for the above question there is no need for the Atomic Number of atom.