Answer:
108.3kPa
Step-by-step explanation:
Given parameters:
Initial temperature = 76k
Initial pressure = 78.4kPa
Final temperature = 105k
Unknown:
Final or new pressure = ?
Solution:
To solve this problem, we apply the combined gas law:
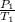 =
=
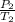
T1 = 76k
P1 = 78.4kPa
T2 = 105k
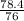 =
=
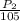
P2 = 108.3atm