Answer : Methanal also known as Formaldehyde
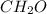 is a chemical Aldehyde which contain ( -CHO) group.
is a chemical Aldehyde which contain ( -CHO) group.
Explanation :
In organic chemistry, a carbonyl group is a functional group which contain a carbon atom double-bonded to an oxygen atom i.e, ( C=O).
If carbonyl group is present in a compound then it can be a carboxylic (RCOOH), aldehyde (RCHO), ketone (RCOR'), ester ((RCOOR') or amide (RCONR'R") group.
Here are some functional groups naming according to the IUPAC rules and image also attached,
Carboxylic acid → (RCOOH) → ( name end in 'OIC ACID' )
Aldehyde → (RCOH) → ( name end in 'AL' )
Ketone → (RCOR') → ( name end in 'ONE' )
Ester → (RCOOR') → ( name end in 'ATE' )
Amide → (RCONR'R") → ( name end in 'AMIDE' )
In an aldehyde, atleast one hydrogen atom must be attached to the carbonyl carbon. For an aldehyde, remove ( -e) from alkane name and add ( -al) at the end of the compound.
Methanal is the IUPAC name for Formaldehyde.