Here we have to calculate the amount of
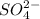 ion present in the sample.
ion present in the sample.
In the sample solution 0.122g of
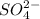 ion is present.
ion is present.
The reaction happens on addition of excess BaCl₂ in a sample solution of potassium sulfate (K₂SO₄) and sodium sulfate [(Na)₂SO₄] can be written as-
K₂SO₄ = 2K⁺ +
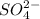
(Na)₂SO₄=2Na⁺ +
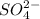
Thus, BaCl₂+
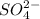 = BaSO₄↓ + 2Cl⁻ .
= BaSO₄↓ + 2Cl⁻ .
(Na)₂SO₄ and K₂SO₄ is highly soluble in water and the precipitation or the filtrate is due to the BaSO₄ only. As a precipitation appears due to addition of excess BaCl₂ thus the total amount of
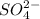 ion is precipitated in this reaction.
ion is precipitated in this reaction.
The precipitate i.e. barium sulfate (BaSO₄)is formed in the reaction which have the mass 0.298g.
Now the molecular weight of BaSO₄ is 233.3 g/mol.
We know the molecular weight of sulfate ion (
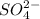 ) is 96.06 g/mol. Thus in 1 mole of BaSO₄ 96.06 g of
) is 96.06 g/mol. Thus in 1 mole of BaSO₄ 96.06 g of
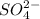 ion is present.
ion is present.
Or. we may write in 233.3 g of BaSO₄ 96.06 g of
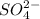 ion is present. So in 1 g of BaSO₄
ion is present. So in 1 g of BaSO₄
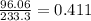 g of
g of
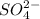 ion is present.
ion is present.
Or, in 0.298 g of the filtered mass (0.298×0.411)=0.122g of
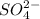 ion is present.
ion is present.