The first order reaction for decomposition of carbon disulfide is as follows:
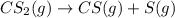
The rate constant for the above reaction is
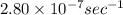 .
.
(a) The expression for rate constant for first order reaction is as follows:
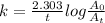
Here, k is rate constant, t is time of the reaction,
![[A_(0)]](https://img.qammunity.org/2019/formulas/physics/college/vnp7p4lqnfvofj9dohr7nisdobp6edklz1.png) is initial concentration of reactant and
is initial concentration of reactant and
![[A_(t)]](https://img.qammunity.org/2019/formulas/physics/college/igtrnnvfmc40l91yok2ptjevfkt41klbyn.png) is concentration at time t.
is concentration at time t.
Here, concentration terms can be replaced by mass now, the mass of reactant at time 37 days should be calculated.
First convert unit of time from days to sec as follows:
1 day=86400 sec
thus,
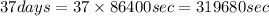
Now, putting the values in expression for rate constant,
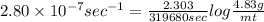
On rearranging,
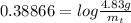
Taking antilog both sides,
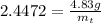
Or,
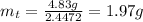
Therefore, mass of carbon disulfide remains after 37 days is 1.97 g.
(b) From the balanced chemical equation for decomposition of carbon disulfide 1 mol of
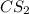 gives 1 mol of CS.
gives 1 mol of CS.
now, mass of carbon disulfide remain after 37 days is 1.97 g thus, mass of carbon disulfide reacted can be calculated as follows:
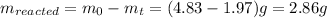
Calculating number of moles from it,
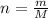
Molar mass of
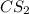 is 76.14 g/mol
is 76.14 g/mol
Putting the values,
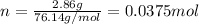
Since, 1 mol of
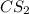 gives 1 mol of CS thus, 0.0375 mol will give 0.0375 mol of CS.
gives 1 mol of CS thus, 0.0375 mol will give 0.0375 mol of CS.
Molar mas of CS is 44.07 g/mol , calculate mass as follows:
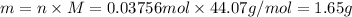
Therefore, mass of carbon monosulfide formed after 37 days is 1.65 g.