The presence of unsaturation that is carbon-carbon double or triple bonds can be tested qualitatively by bromine test. In this test, the unknown sample is treated with a small amount of elemental bromine in organic solvent (which is deep brown in color). The disappearance of deep brown color of bromine results due to reaction of bromine with unsaturated sample.
The reaction of eugenol with bromine is given as:
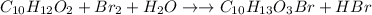
Since the number of atoms of each element in the reactant side is equal to the number of atoms of elements of product side. Thus, the reaction is balanced.
The reaction between eugenol and bromine is shown in the image.