Answer:
784.4kg
Step-by-step explanation:
Given parameters:
Mass of Mg = 200kg
Unknown:
Mass of MgCl₂ = ?
Solution:
The reaction equation is given as:
Mg + Cl₂ → MgCl₂
Find the number of moles of the given specie;
Number of moles of Mg =
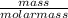 =
=
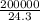 = 8230.5mole
= 8230.5mole
So;
1 mole of Mg will produce 1 mole of MgCl₂
8230.5mole of Mg will produce 8230.5mole of MgCl₂
Mass of MgCl₂ = number of moles x molar mass
Molar mass of MgCl₂ = 24.3 + 2(35.5) = 95.3g/mol
Mass of MgCl₂ = 8230.5mole x 95.3g/mol = 784.4kg