Answer:
63.09 kJ is the quantity of heat that is released when 27.9 g of water condenses.
Step-by-step explanation:
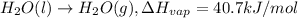
Latent heat of vaporization =
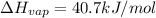
Amount of heat required to condense 1 mole of water = 40.7 kJ
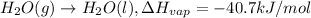
Mass of water given = 27.9 g
Moles of water :
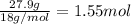
Heat required to vaporize 1.55 moles of water:
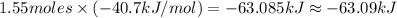
Negative sign indicates that energy will release.