Answer: Option (C) is the chemical reaction.
Step-by-step explanation:
A chemical reaction is defined as the reaction which leads to the formation of a new compound or substance due to exchange of valence electrons of the reactants.
For example,
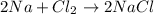
Here, sodium donates its one valence electron to a chlorine atom in order to become stable. Therefore, an ionic bond is formed and a new compound NaCl is formed as a result of this chemical reaction.
Whereas number of atoms and elements involved in a chemical reaction remains the same.
Thus, we can conclude that the chemical bonds between atoms is altered during a chemical reaction.