Answer : The correct option is, (B) 5.23
Explanation :
pH : It is defined as the negative logarithm of hydrogen ion concentration.
Mathematically, it is represented as :
![pH=-\log [H^+]](https://img.qammunity.org/2019/formulas/physics/high-school/iidawris7irvi0bu33z0a9xjzagtaknk6o.png)
First we have to calculate the pH.
![pH=-\log [H^+]](https://img.qammunity.org/2019/formulas/physics/high-school/iidawris7irvi0bu33z0a9xjzagtaknk6o.png)
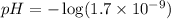
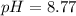
Now we have to calculate the pOH.
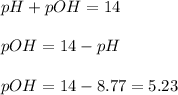
Therefore, the pOH of the solution is, 5.23