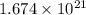Answer: The number of atoms in given amount of cocaine hydrochloride is
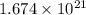
Step-by-step explanation:
To calculate the number of moles, we use the equation:
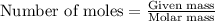
Given mass of cocaine hydrochloride = 21.0 mg = 0.021 g (Conversion factor: 1 g = 1000 mg)
Molar mass of cocaine hydrochloride = 339.8 g/mol
Putting values in above equation, we get:
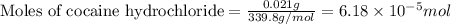
In 1 mole of cocaine hydrochloride, 17 moles of carbon atom, 22 moles of hydrogen atom, 1 mole of chlorine atom, 1 mole of nitrogen atom and 4 moles of oxygen atoms are present
Total number of atoms in 1 mole of cocaine hydrochloride = (17 + 22 + 1 + 1 + 4) = 45 atoms
According to mole concept:
1 mole of a substance contains
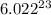 number of atoms.
number of atoms.
So,
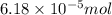 of cocaine hydrochloride will contain =
of cocaine hydrochloride will contain =
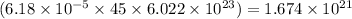 number of atoms
number of atoms
Hence, the number of atoms in given amount of cocaine hydrochloride is