Answer: The species that are present in a solution of sodium perchlorate are
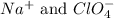 ions.
ions.
Step-by-step explanation:
We are given:
An ionic compound having chemical formula
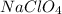
Ionic compound is defined as the compound which is formed when electron gets transferred from one atom to another atom. These are usually formed when a metal reacts with a non-metal or a metal reacts with a polyatomic ion or a reaction between two polyatomic ions takes place.
This chemical compound is formed by the combination of sodium ions and perchlorate ions.
When sodium perchlorate is dissolved in water, it dissociates into its ions.
The chemical equation for the ionization of sodium perchlorate follows:
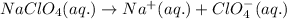
Hence, the species that are present in a solution of sodium perchlorate are
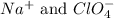 ions.
ions.