Answer : The density of sample of liquid is 1.28 g/mL
Explanation :
Density : It is defined as the mass contained per unit volume.
Formula used :
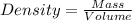
Given :
Mass of sample of liquid = 0.16 kg = 160 g
conversion used : (1 kg = 1000 g)
Volume of sample of liquid = 125 mL
Now put all the given values in the above formula, we get:
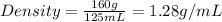
Therefore, the density of sample of liquid is 1.28 g/mL