Answer:- 448 mL of hydrogen gas are formed.
Solution:- It asks to calculate the volume of hydrogen gas formed in milliliters at STP when 0.020 moles of magnesium reacts with excess HCl acid. The balanced equation is:

There is 1:1 mol ratio between Mg and hydrogen gas. So, the moles of hydrogen gas is also equals to the moles of Mg reacted.
moles of Hydrogen gas formed = 0.020 mol
At STP, volume of 1 mol of the gas is 22.4 L. We need to calculate the volume of 0.02 moles of hydrogen gas.
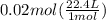
= 0.448 L
They want answer in mL. So, let's convert L to mL using the conversion formula, 1L = 1000mL
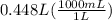
= 448 mL
So, 0.020 moles of magnesium would produce 448 mL of hydrogen gas at STP on reacting with excess of HCl acid.