Answer:- First choice is correct. Empirical formula is
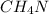 .
.
Solution:- Mass percentages are given for C, H and N for a compound and we are asked to find out it's empirical formula. First of all we calculate the moles of each element on dividing it's percentage by it's atomic mass;
C =
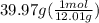 = 3.33
= 3.33
H =
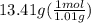 = 13.3
= 13.3
N =
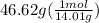 = 3.33
= 3.33
Let's divide the moles of each by the least one of them which is 3.33.
So, C =
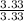 = 1
= 1
H =
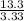 = 4
= 4
N =
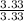 = 1
= 1
Hence, the empirical formula of the compound is
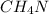 . First choice is correct.
. First choice is correct.