Answer:- molecular formula is
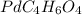 .
.
Solution:- Percentages are given from which we will calculate the moles of each element.
moles of Pd =
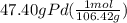 = 0.445 mol Pd
= 0.445 mol Pd
moles of O =
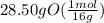 = 1.78 mol O
= 1.78 mol O
moles of C =
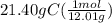 = 1.78 mol C
= 1.78 mol C
moles of H =
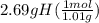 = 2.66 mol H
= 2.66 mol H
Now we divide the moles of each by the least one of them. The least one is 0.445. So, let's divide the moles of each by 0.445.
Pd =
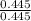 = 1
= 1
O =
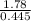 = 4
= 4
C =
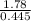 = 4
= 4
H =
 = 6
= 6
So, the molecular formula of the compound is
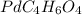 .
.