Answer:
i. 3 moles.
ii. 132.03 grams.
iii. 105.6 grams.
Step-by-step explanation:
Hello!
In this case, given the balanced chemical reaction, we can proceed as follows:
i. By starting with 5 moles of oxygen, via the 5:3 mole ratio we compute the produced moles of CO2:
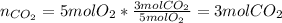
ii. Now, since we have previously computed the moles of CO2 from the same moles of oxygen, by using its molar mass (44.02 g/mol), we obtain:
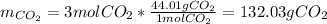
iii. Now, we need to combine the previously used two proportional factors for the calculation of the mass of CO2 from 128.00 grams of oxygen (molar mass 32.00 g/mol):
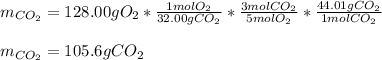
Best regards!