Answer:
The pressure of the gas is 0.35 atm.
Step-by-step explanation:
We can find the pressure of the gas by using the Ideal Gas Law:
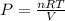
Where:
V: is the volume = 605 mL = 0.605 L
n: is the number of moles = 0.00803 moles
T: is the temperature = 39 °C = 312 K
R: is the gas constant = 0.082 L*atm/(K*mol)
Hence, the pressure is:
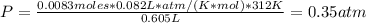
Therefore, the pressure of the gas is 0.35 atm.
I hope it helps you!