Answer:
b.The 2s orbital in He atom is less stable than 2s orbital in He+.
Step-by-step explanation:
We have to find incorrect comparison
1.The 1 s orbital in H is more stable than the 1 s orbital in He+
We know that
Atomic number of H=1
Atomic number of He+=1
Configuration of H=
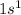
Configuration of He+=
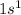
We know that neutral atom is more stable than charged atom.
He atom have two electron before losing one electron but after losing 1 electron it becomes positive ion.
Both have half filled configuration but helium atom attained this configuration after losing on electron.
So, it highly reactive to fill their outer shell.
Therefore, it is less stable.
Therefore, the 1 s orbital of H is more stable than the 1 s orbital of He+.
b.The 2s orbital in He atom is le.ss stable than 2s orbital in He+.
Configuration He=
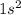
Configuration of He+=
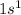
We know that fully filled or half filled configuration are stable configuration.
Configuration of He is full filled because s orbital can have maximum two electron .
But both cannot have 2 s orbital .
If atom have no 2s orbital then we can not compare stability of 2s orbital of He and He+.
So , it is false.
C.The 2s sub-shell in Li is more stable than the 2p sub-shell in Li.
Atomic number of Li=3
Configuration of Li=
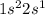
We know that s orbital can have maximum 2 electron and p orbital can have maximum 6 electrons.
Half filled or fully filled configuration is more stable configuration .
2s orbital of Li is half filled .Therefore, it is more stable.
Hence, it is true.
Answer:b.The 2s orbital in He atom is less stable than 2s orbital in He+.