solution:
The formula to calculate Avagadro’s number(N) from density the following formula is used
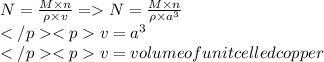
N=Avagadro number
M=molar mass of copper
N=number of copper atoms occupying one unit cell of copper
Delta=Density of copper
A= length of edge of unit cell of copper.
Hence from the given option (a),(b),(c) are required. Only atomic number of copper is not needed.
Hence the correct answer is atomic number of copper