From the given balanced equation we have find out the amount (in gm) of Ag formed from 5.50 gm of Ag₂O.
2Ag₂O(s) → 4Ag (s) + O₂ (g)
We know, molecular mass of Ag₂O= 231.7 g/mol, and atomic mass of Ag= 107.8 g/mol. Given, mass of Ag₂O=5.50 gm. Number moles of Ag₂O=
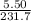 = 0.0237 moles.
= 0.0237 moles.
From the balanced chemical reaction we get 2 (two) moles of Ag₂O produces 4 (four) moles of Ag. So, 0.0237 moles of Ag₂O produces
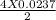 moles=0.0474 moles of Ag= 0.0474 X 107.8 g of Ag=5.11g Ag.
moles=0.0474 moles of Ag= 0.0474 X 107.8 g of Ag=5.11g Ag.
Therefore, 5.50 g Ag₂O produces 5.11 g of Ag as per the given balanced chemical reaction.