Answer : The standard internal energy of formation of liquid methyl acetate is, -432.1 kJ/mol
Explanation :
The balanced reaction of formation of liquid methyl acetate will be:
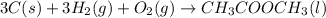
Formula used :
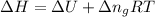
or,
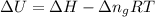
where,
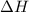 = change in enthalpy =
= change in enthalpy =
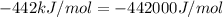
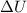 = change in internal energy = ?
= change in internal energy = ?
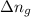 = change in moles = 0 - 4 = -4 (from the reaction)
= change in moles = 0 - 4 = -4 (from the reaction)
R = gas constant = 8.314 J/mol.K
T = temperature = 298 K
Now put all the given values in the above formula, we get:
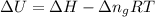
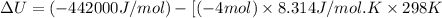
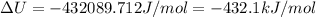
Therefore, the standard internal energy of formation of liquid methyl acetate is, -432.1 kJ/mol