Answer: 501900 calories.
Step-by-step explanation:
Exothermic processes are those processes in which heat is released and endothermic processes are those processes in which heat is absorbed.
Standard units for heat measurement are Joules and calories wherein
1 joule = 0.239 Calorie.
Now 1 kJ = 1000 Joules
Therefore 2100kJ =
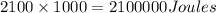
As 1 Jolule is equivalent to 0.239 calorie
2100000 Joules are equivalent to=
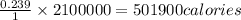
Thus it is 501900 calories.