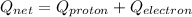Answer:
total number of electrons required = 3
Step-by-step explanation:
Lithium atom has 3 protons inside its nucleus
so the net positive charge on atom is due to these 3 protons
so in order to make it neutral we need same amount of negative charge on it so that net charge becomes zero on an atom of lithium
So we need 3 electrons on atom of nucleus as we know that
charge of proton = +e
charge of electron = -e
so we will have
![Q_(proton) = +3e{/tex]</p><p>[tex]Q_(electron) = -3e](https://img.qammunity.org/2019/formulas/physics/high-school/wmif2ov8vauxdxa9lutalf2bm0ja139g0a.png)