Answer : % ionic character is 0.20%
Bonding between the two metals will be purely metallic.
Step-by-step explanation: For the calculation of % ionic character, we use the formula
![\% \text{ ionic character}= [1-e^{(-(X_A-X_B)^2))/(4)}]*(100\%)](https://img.qammunity.org/2019/formulas/chemistry/college/u29stkasvg9b8nkayrtsh45c26gxi2vwuq.png)
where
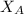 &
&
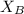 are the Pauling's electronegativities.
are the Pauling's electronegativities.
The table attached has the values of electronegativities, by taking the values of Al and Mn from there,
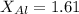
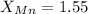
Putting the values in the electronegativity formula, we get
![\% \text{ ionic character}= [1-e^{(-(1.61-1.55)^2))/(4)}]*(100\%)](https://img.qammunity.org/2019/formulas/chemistry/college/5eepald92bgmi4dfrjg4qna0vxhyqbx6og.png)
= 0.20%
Now, there are 3 types of inter atomic bonding
1) Ionic Bonding: It refers to the chemical bond in which there is complete transfer of valence electrons between atoms
2) Covalent Bonding: It refers to the chemical bond involving the sharing of electron pairs between 2 atoms.
3) Metallic Bonding: It refers to the chemical bond in which there is an electrostatic force between the positively charged metal ions and delocalised electrons.
In
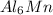 compound, the % ionic character is minimal that is
compound, the % ionic character is minimal that is
 0.20% and there are two metal ions present, therefore this compound will have metallic bonding.
0.20% and there are two metal ions present, therefore this compound will have metallic bonding.