Answer:
0.35215 grams of silver chloride required to plate 265 mg of pure silver.
Step-by-step explanation:
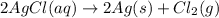
Mass of silver = 265 mg = 0.265 g
Moles of silver =
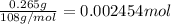
According to reaction, 2 moles of silver are obtained from 2 moles of silver chloride.
Then 0.002454 moles of silver will be obtained from :
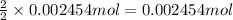 of silver chloride
of silver chloride
Mass of 0.002454 moles of silver chloride:
= 0.002454 mol × 143.5 g/mol = 0.35215 g
0.35215 grams of silver chloride required to plate 265 mg of pure silver.