Answer:- 47.62 mL
Solution:- It is a dilution problem where we are asked to calculate the volume of 15.75 M perchloric acid solution required to make 500.0 mL of 1.500 M solution.
For solving this type of problems we use the dilution equation:
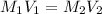
Where,
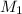 is the concentration of the concentrated solution and
is the concentration of the concentrated solution and
 is it's volume.
is it's volume.
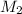 is the concentration of the diluted solution and
is the concentration of the diluted solution and
 is it's volume. Let's plug in the values in the equation and solve it for
is it's volume. Let's plug in the values in the equation and solve it for
 .
.
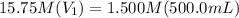
On rearranging this for
 :
:
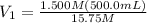
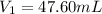
So, 47.62 mL of 15.75 M perchloric acid are required to make 500.0mL of 1.500 M solution.