Answer:- 1.15 tons of sulfur dioxide will be produced.
Solution:- From given information, 25 tons of coal has 2.30% sulfur by mass. It means 100 g of sample sample has 2.30 grams of sulfur. This sulfur is converted to sulfur dioxide on combustion.
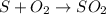
From balanced equation, 32 g of sulfur(atomic mass) gives 64 g of sulfur dioxide(molar mass) .
We would dimensional analysis of solving this problem.
1 ton = 1000 kg
1 kg = 1000 g
let's make the set up as:
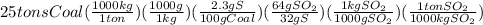
=

So, 25 tons of coal sample will produce 1.15 tons of sulfur dioxide.