When an object gets heated by a temperature ΔT energy needed, E = mcΔT
Here energy is given E = 2050 J
Mass of object = 150 g
Change in temperature ΔT = 15
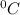 = 15 K
= 15 K
a) Heat capacity of an object equal to the ratio of the heat added to (or removed from) an object to the resulting temperature change.
So heat capacity = E/ΔT = 2050/15 = 136.67 J/K
b) We have E = mcΔT
c =
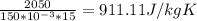
So object's specific heat = 911.11 J/kgK