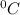Taking specific heat of lead as 0.128 J/gK = c
We have energy of ball at 7.00 meter height = mgh =
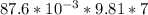
When leads gets heated by a temperature ΔT energy needed = mcΔT
=
 ΔT
ΔT
Comparing both the equations
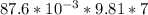 =
=
 ΔT
ΔT
ΔT = 0.536 K
Change in temperature same in degree and kelvin scale
So ΔT = 0.536