Answer: D) It has two more electrons than protons.
Step-by-step explanation: Sulphur (S) is an element with atomic number 16 and thus contains 16 electrons. A neutral atom contains equal number of protons and electrons.
![S:16:[Ne]3s^23p^4](https://img.qammunity.org/2019/formulas/chemistry/middle-school/iolgjq4jbv9fwj7npnhyzae8s6rbsex7lg.png)
An atom on losing electrons gains positive charge and on gaining electrons gain negative charge.
Sulphide ion
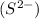 is formed by gaining 2 electrons to attain stable configuration of argon and thus contains two more electrons than protons.
is formed by gaining 2 electrons to attain stable configuration of argon and thus contains two more electrons than protons.
![S^(2-):18:[Ne]3s^23p^6:[Ar]](https://img.qammunity.org/2019/formulas/chemistry/middle-school/svznsob0qo0czj0l0z1fogpve5bxplal5o.png)