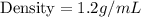Answer:
The density of mineral is:
1.2 g/mL
Explanation:
It is given that:
when a mineral with mass 9.6 g is placed in a cylinder with 8.0 mL of water then the water level rises to: 16.0 mL
This means that:
The volume of mineral is:
Volume of the water level after the mineral-Original volume of water in cylinder.
= 16-8
= 8 mL
Now, we know that the density of a substance is the ratio of mass to volume .
i.e.
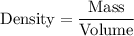
Hence, the density of substance will be:
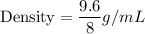
i.e.