Answer:
Density of mineral is 1.2
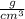 .
.
Given:
mass = 9.6 gram
Initial volume = 8 ml
Final volume = 16 ml
To find:
Density of the mineral = ?
Formula used:
density =
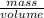
Solution:
According to Archimedes principle the volume of liquid displace is equal to the volume of mineral.
volume of mineral = 16 ml - 8 ml = 8 ml = 8
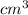
Density of the mineral is given by,
density =
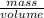
density =
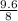
density = 1.2
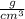
Density of mineral is 1.2
 .
.