The compound contain only carbon, hydrogen and oxygen, let the empirical formula of compound be
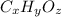 .
.
The mass of compound, carbon dioxide and water is 43.9 mg, 119 mg and 24.4 mg respectively . The molar mass of compound, carbon dioxide and water is 162 g/mol, 44.01 g/mol and 18 g/mol respectively.
Converting the mass into number of moles as follows:
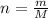
Here, m is mass and M is molar mass.
Number of moles of carbon dioxide
 will be:
will be:
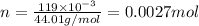
1 mol of
 has 1 mol of C thus, number of moles of C from 0.0027 mol of
has 1 mol of C thus, number of moles of C from 0.0027 mol of
 will be 0.0027 mol.
will be 0.0027 mol.
Molar mass of C is 12 g/mol thus, mass of C will be:
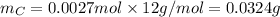
Number of moles of water
 will be:
will be:
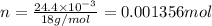
1 mol of
 has 2 mol of H thus, 0.001356 mol of
has 2 mol of H thus, 0.001356 mol of
 will have
will have
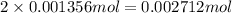 mole of H.
mole of H.
Molar mass of H is 1 g/mol thus, mass of H will be:
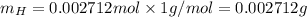
Sum of mass of C and H will be:
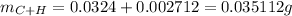
Mass of compound is 43.9 mg or 0.0439 g thus, mass of oxygen will be:
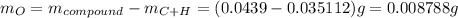
Molar mass of oxygen is 16 g/mol thus, number of moles of oxygen will be:
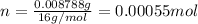
Taking the molar ratio:
C:H:O=0.0027:0.002712:0.00055=5:5:1
Therefore, empirical formula will be
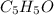 .
.
The molar mass of
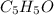 is 81 g/mol
is 81 g/mol
The molar mass of given compound is 162 that is 2 times the molar mass calculated above thus, molecular formula will be
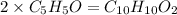 .
.
Therefore, empirical formula and molecular formula of the compound is
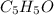 and
and
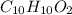 respectively.
respectively.