Molarity of concentrated nitric acid = 15.9 M
Volume of the stock solution to be prepared = 500.0 mL
Concentration of the stock that is to be prepared = 0.750 M
Calculating moles from molarity and volume of stock:
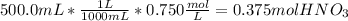
Calculating volume of concentrated nitric acid to be taken for the preparation of stock solution:
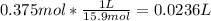
Converting L to mL:
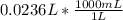 = 23.6 mL
= 23.6 mL
Volume of distilled water to be added to 23.6 mL of 15.9 M nitric acid to get the given concentration = 5000.0mL-23.6mL=976.4 mL
Therefore, 976.4 mL distilled water is to be added to 23.6 mL of 15.9 M nitric acid solution to prepare 500.0 mL of 0.750M nitric acid.